Chapter 8 Beta diversity
Beta diversity is another name for sample dissimilarity. It quantifies differences in the overall taxonomic composition between two samples.
Common indices include Bray-Curtis, Unifrac, Jaccard index, and the Aitchison distance. Each of these (dis)similarity measures emphasizes different aspects. For example, UniFrac incorporates phylogenetic information, and Jaccard index ignores exact abundances and considers only presence/absence values. For more background information and examples, you can check the dedicated section in online book.
8.1 Examples of PCoA with different settings
Beta diversity estimation generates a (dis)similarity matrix that contains for each sample (rows) the dissimilarity to any other sample (columns).
This complex set of pairwise relations can be visualized in informative ways, and even coupled with other explanatory variables. As a first step, we compress the information to a lower dimensionality, or fewer principal components, and then visualize sample similarity based on that using ordination techniques, such as Principal Coordinate Analysis (PCoA). PCoA is a non-linear dimension reduction technique, and with Euclidean distances it is is identical to the linear PCA (except for potential scaling).
We typically retain just the two (or three) most informative top components, and ignore the other information. Each sample has a score on each of these components, and each component measures the variation across a set of correlated taxa. The top components are then easily visualized on a two (or three) dimensional display.
Let us next look at some concrete examples.
8.1.1 PCoA for ASV-level data with Bray-Curtis
Let us start with PCoA based on a Bray-Curtis dissimilarity matrix calculated at Genus level abundances.
# Pick the relative abundance table
rel_abund_assay <- assays(tse)$relabundance
# Calculates Bray-Curtis distances between samples. Because taxa is in
# columns, it is used to compare different samples. We transpose the
# assay to get taxa to columns
bray_curtis_dist <- vegan::vegdist(t(rel_abund_assay), method = "bray")
# PCoA
bray_curtis_pcoa <- ecodist::pco(bray_curtis_dist)
# All components could be found here:
# bray_curtis_pcoa$vectors
# But we only need the first two to demonstrate what we can do:
bray_curtis_pcoa_df <- data.frame(pcoa1 = bray_curtis_pcoa$vectors[,1],
pcoa2 = bray_curtis_pcoa$vectors[,2])
# Create a plot
bray_curtis_plot <- ggplot(data = bray_curtis_pcoa_df, aes(x=pcoa1, y=pcoa2)) +
geom_point() +
labs(x = "PC1",
y = "PC2",
title = "Bray-Curtis PCoA") +
theme(title = element_text(size = 10)) # makes titles smaller
bray_curtis_plot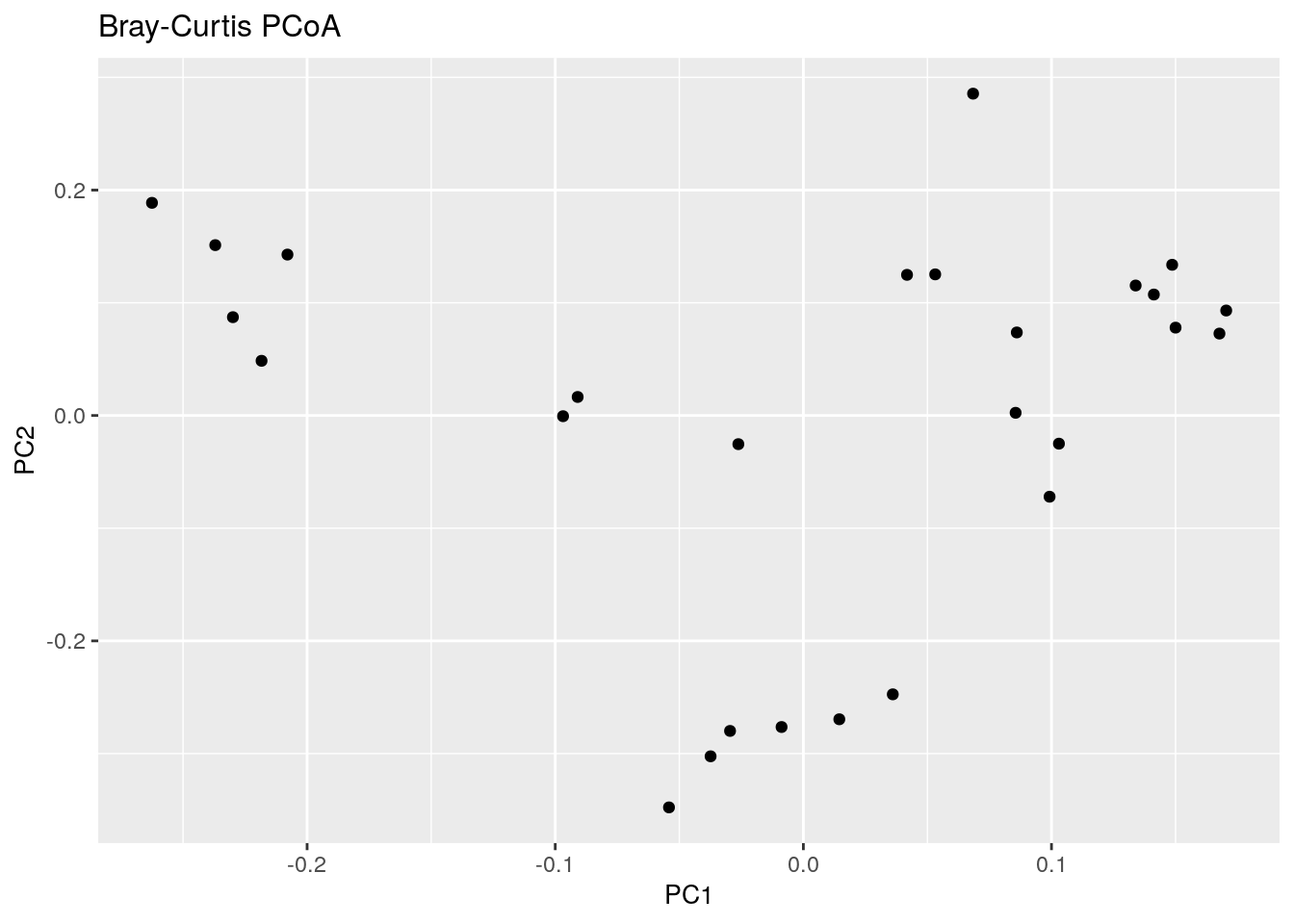
8.1.2 PCoA for ASV-level data with Aitchison distance
Now the same using Aitchison distance. This metric corresponds to Euclidean distances between CLR transformed sample abundance vectors.
# Does clr transformation. Pseudocount is added, because data contains zeros.
tse <- transformCounts(tse, method = "clr", pseudocount = 1)
# Gets clr table
clr_assay <- assays(tse)$clr
# Transposes it to get taxa to columns
clr_assay <- t(clr_assay)
# Calculates Euclidean distances between samples. Because taxa is in columns,
# it is used to compare different samples.
euclidean_dist <- vegan::vegdist(clr_assay, method = "euclidean")
# Does principal coordinate analysis
euclidean_pcoa <- ecodist::pco(euclidean_dist)
# Creates a data frame from principal coordinates
euclidean_pcoa_df <- data.frame(pcoa1 = euclidean_pcoa$vectors[,1],
pcoa2 = euclidean_pcoa$vectors[,2])
# Creates the plot
euclidean_plot <- ggplot(data = euclidean_pcoa_df, aes(x=pcoa1, y=pcoa2)) +
geom_point() +
labs(x = "PC1",
y = "PC2",
title = "Euclidean PCoA with CLR transformation") +
theme(title = element_text(size = 12)) # makes titles smaller
euclidean_plot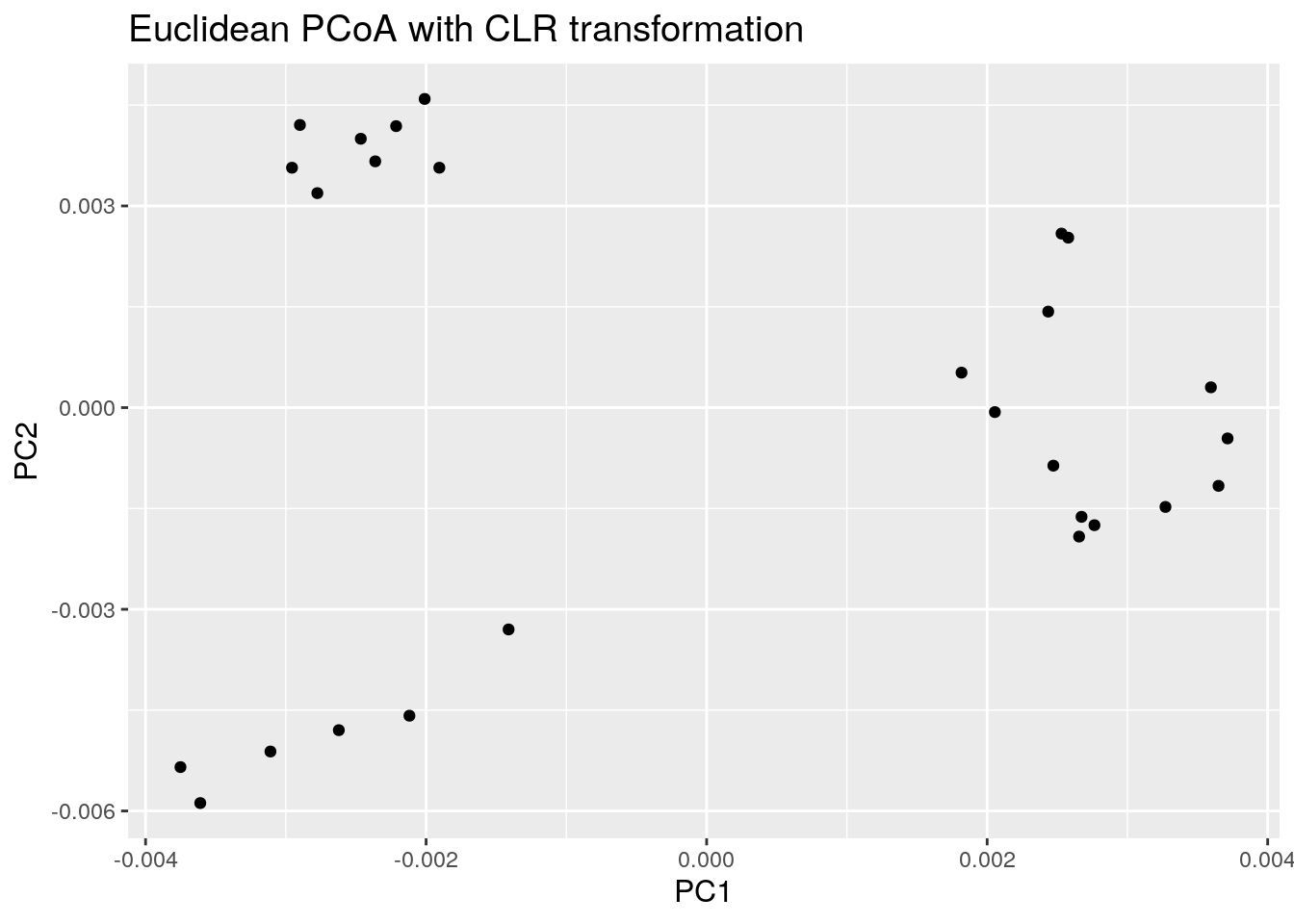
8.1.3 PCoA aggregated to Phylum level
We use again the Aitchison distances in this example but this time applied to the phylum level.
# Does clr transformation. Psuedocount is added, because data contains zeros.
tse_phylum <- transformCounts(tse_phylum, method = "clr", pseudocount = 1)
# Gets clr table
clr_phylum_assay <- assays(tse_phylum)$clr
# Transposes it to get taxa to columns
clr_phylum_assay <- t(clr_phylum_assay)
# Calculates Euclidean distances between samples. Because taxa is in columns,
# it is used to compare different samples.
euclidean_phylum_dist <- vegan::vegdist(clr_assay, method = "euclidean")
# Does principal coordinate analysis
euclidean_phylum_pcoa <- ecodist::pco(euclidean_phylum_dist)
# Creates a data frame from principal coordinates
euclidean_phylum_pcoa_df <- data.frame(
pcoa1 = euclidean_phylum_pcoa$vectors[,1],
pcoa2 = euclidean_phylum_pcoa$vectors[,2])
# Creates a plot
euclidean_phylum_plot <- ggplot(data = euclidean_phylum_pcoa_df,
aes(x=pcoa1, y=pcoa2)) +
geom_point() +
labs(x = "PC1",
y = "PC2",
title = "Aitchison distances at Phylum level") +
theme(title = element_text(size = 12)) # makes titles smaller
euclidean_phylum_plot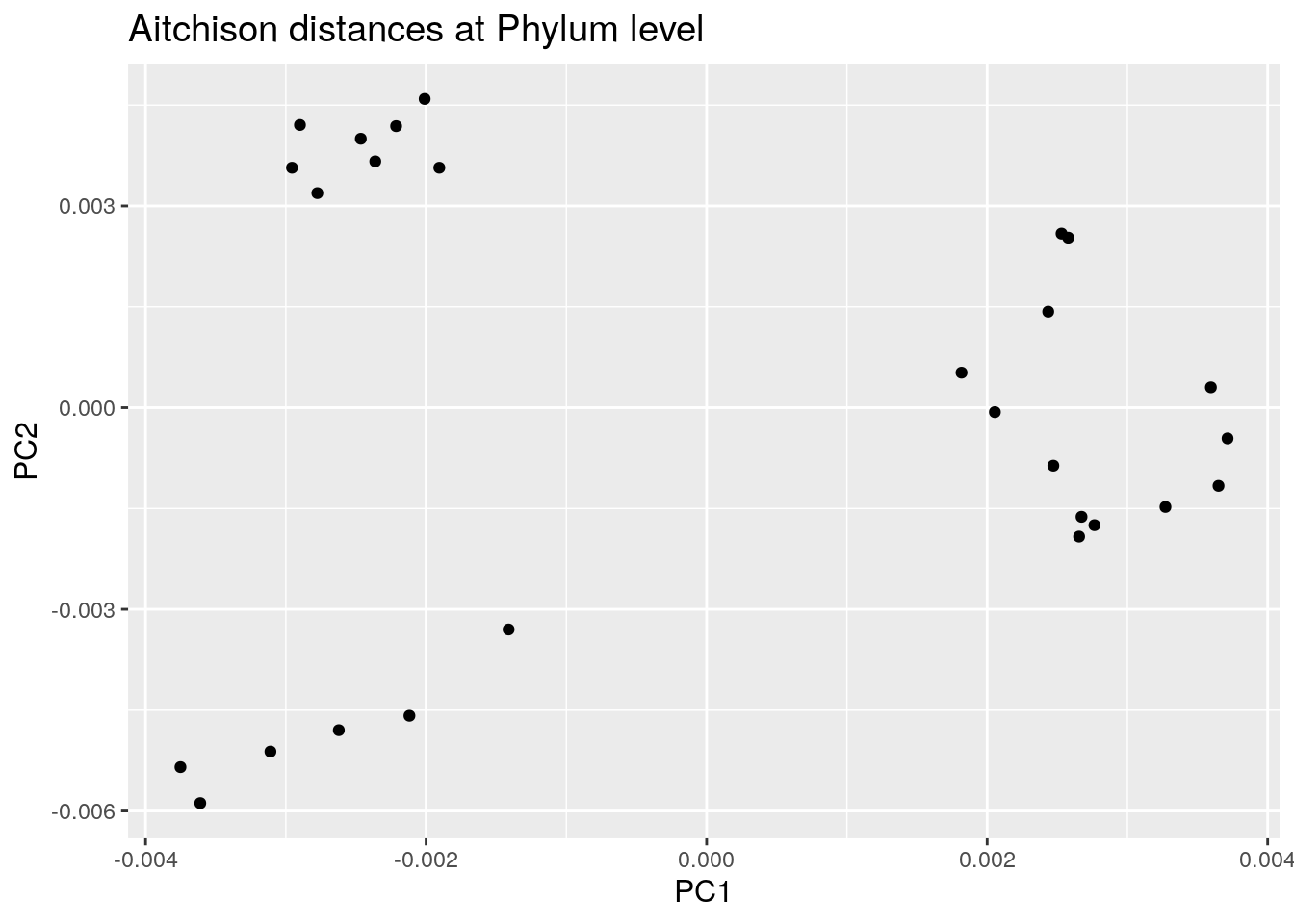
8.2 Highlighting external variables
We can map other variables on the same plot for example by coloring the points accordingly.
8.2.1 Discrete grouping variable shown with colors
# Adds the variable we later use for coloring to the data frame
euclidean_patient_status_pcoa_df <- cbind(euclidean_pcoa_df,
patient_status = colData(tse)$patient_status)
# Creates a plot
euclidean_patient_status_plot <- ggplot(data = euclidean_patient_status_pcoa_df,
aes(x=pcoa1, y=pcoa2,
color = patient_status)) +
geom_point() +
labs(x = "PC1",
y = "PC2",
title = "PCoA with Aitchison distances") +
theme(title = element_text(size = 12)) # makes titles smaller
euclidean_patient_status_plot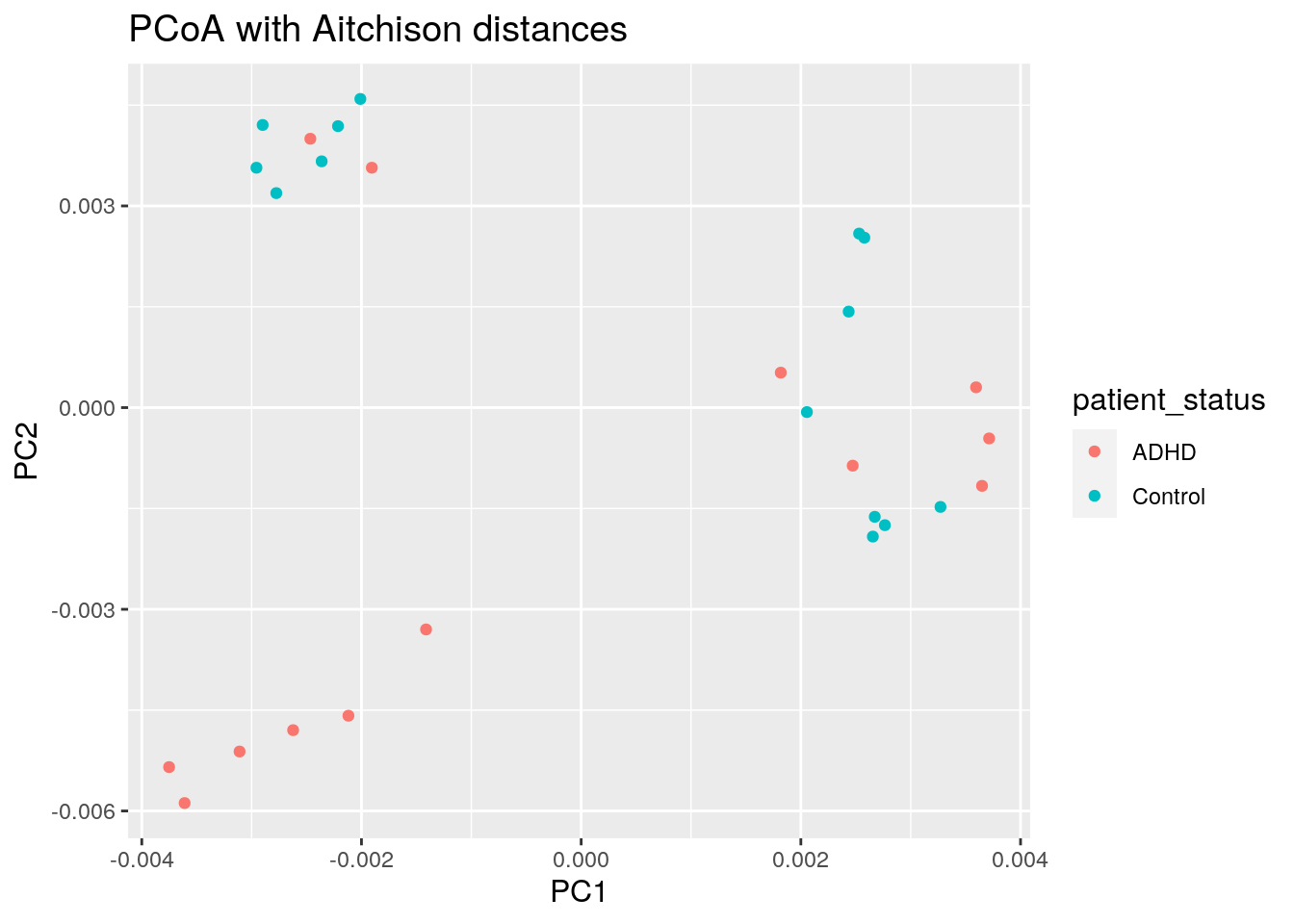
8.2.2 PCoA plot with continuous variable
We can do the same as above using any continuous variable. E.g. let us see how the plotted samples differ in their alpha diversity (as an example of a continuous variable):
# Adds coloring information to the data frame, creates new column
euclidean_shannon_pcoa_df <- cbind(euclidean_pcoa_df,
shannon = colData(tse)$Shannon_diversity)
# Creates a plot
euclidean_shannon_plot <- ggplot(data = euclidean_shannon_pcoa_df,
aes(x=pcoa1, y=pcoa2,
color = shannon)) +
geom_point() +
labs(x = "PC1",
y = "PC2",
title = "PCoA with Aitchison distances") +
theme(title = element_text(size = 12)) # makes titles smaller
euclidean_shannon_plot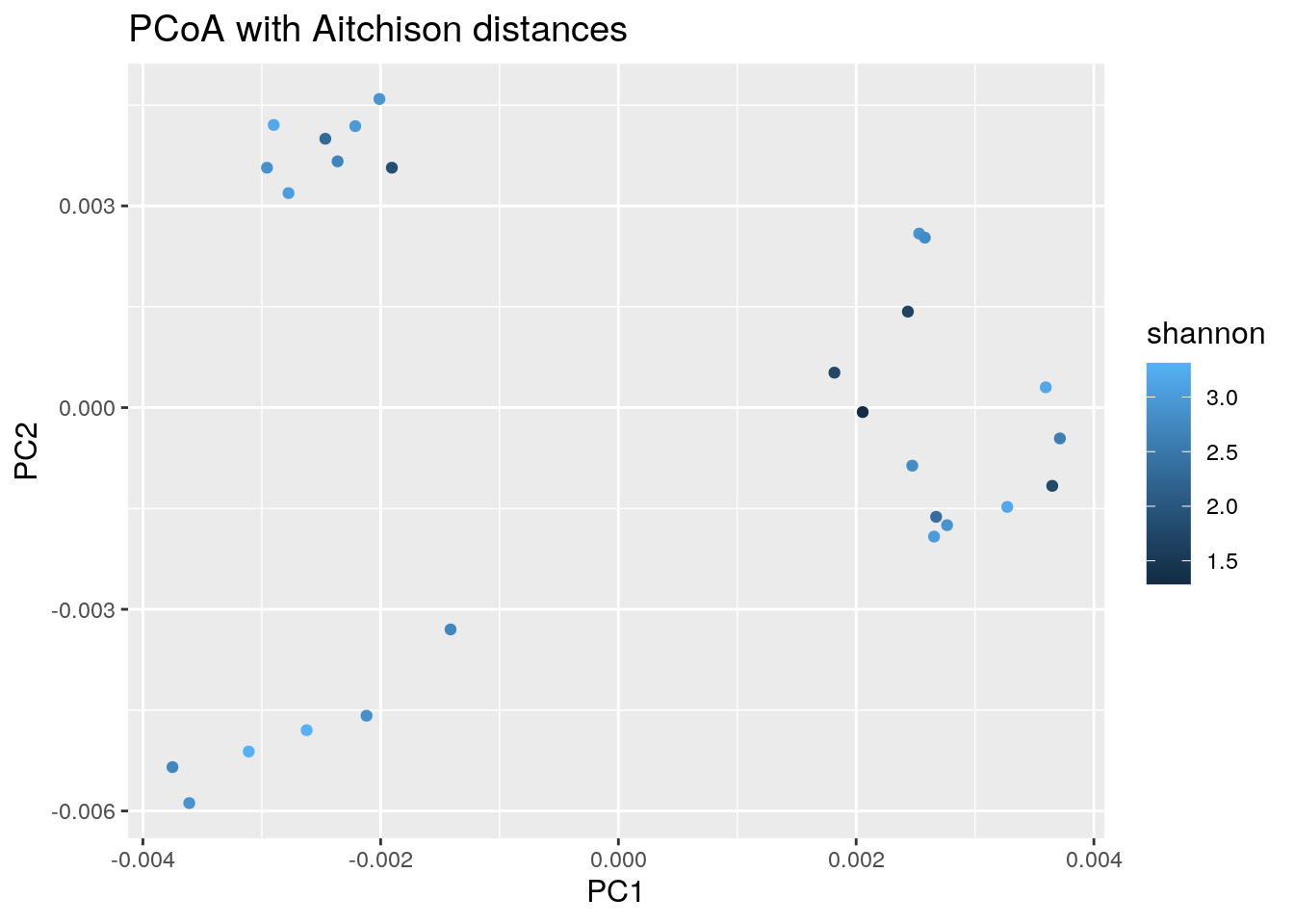
8.3 Estimating associations with an external variable
Next to visualizing whether any variable is associated with differences between samples, we can also quantify the strength of the association between community composition (beta diversity) and external factors.
The standard way to do this is to perform a so-called permutational multivariate analysis of variance (PERMANOVA). This method takes as input the abundance table, which measure of distance you want to base the test on and a formula that tells the model how you think the variables are associated with each other.
# First we get the relative abundance table
rel_abund_assay <- assays(tse)$relabundance
# again transpose it to get taxa to columns
rel_abund_assay <- t(rel_abund_assay)
# then we can perform the method
permanova_cohort <- vegan::adonis(rel_abund_assay ~ cohort,
data = colData(tse),
permutations = 9999)
# we can obtain a the p value for our predictor:
print(paste0("Different different cohorts and variance of abundance ",
"between samples, p-value: ",
as.data.frame(permanova_cohort$aov.tab)["cohort", "Pr(>F)"]))## [1] "Different different cohorts and variance of abundance between samples, p-value: 0.7382"The cohort variable is not significantly associated with microbiota composition (p-value is over 0.05).
We can, however, visualize those taxa whose abundances drive the differences between cohorts. We first need to extract the model coefficients of taxa:
# Gets the coefficients
coef <- coefficients(permanova_cohort)["cohort1",]
# Gets the highest coefficients
top.coef <- sort(head(coef[rev(order(abs(coef)))],20))
# Plots the coefficients
top_taxa_coeffient_plot <- ggplot(data.frame(x = top.coef,
y = factor(names(top.coef),
unique(names(top.coef)))),
aes(x = x, y = y)) +
geom_bar(stat="identity") +
labs(x="", y="", title="Top Taxa") +
theme_bw()
top_taxa_coeffient_plot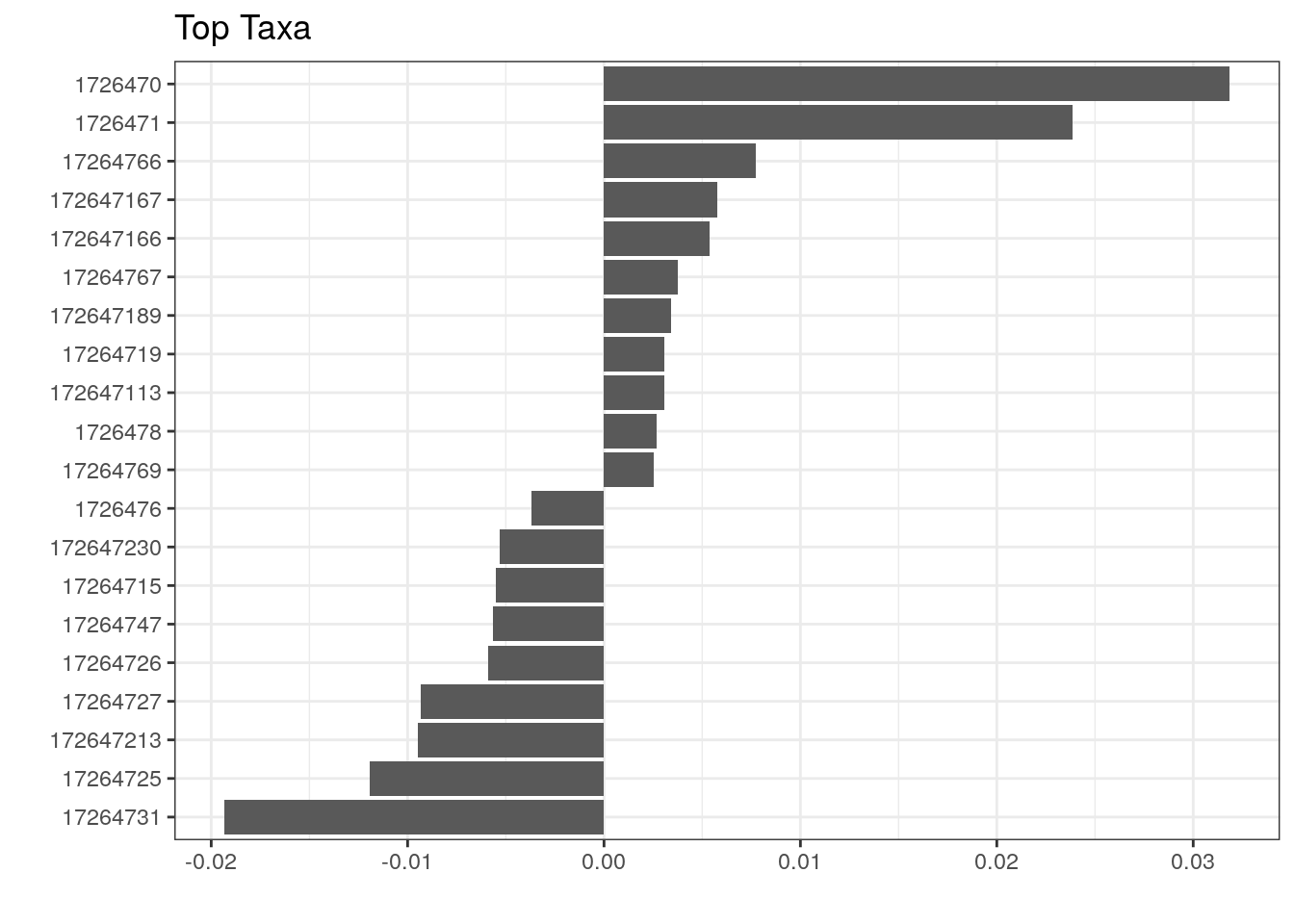
The above plot shows taxa as code names, and it is hard to tell which bacterial groups they represent. However, it is easy to add human readable names. We can fetch those from our rowData. Here we use Genus level names:
# Gets corresponding Genus level names and stores them to top.coef
names <- rowData(tse)[names(top.coef), ][,"Genus"]
# Adds new labels to the plot
top_taxa_coeffient_plot <- top_taxa_coeffient_plot +
scale_y_discrete(labels = names) # Adds new labels
top_taxa_coeffient_plot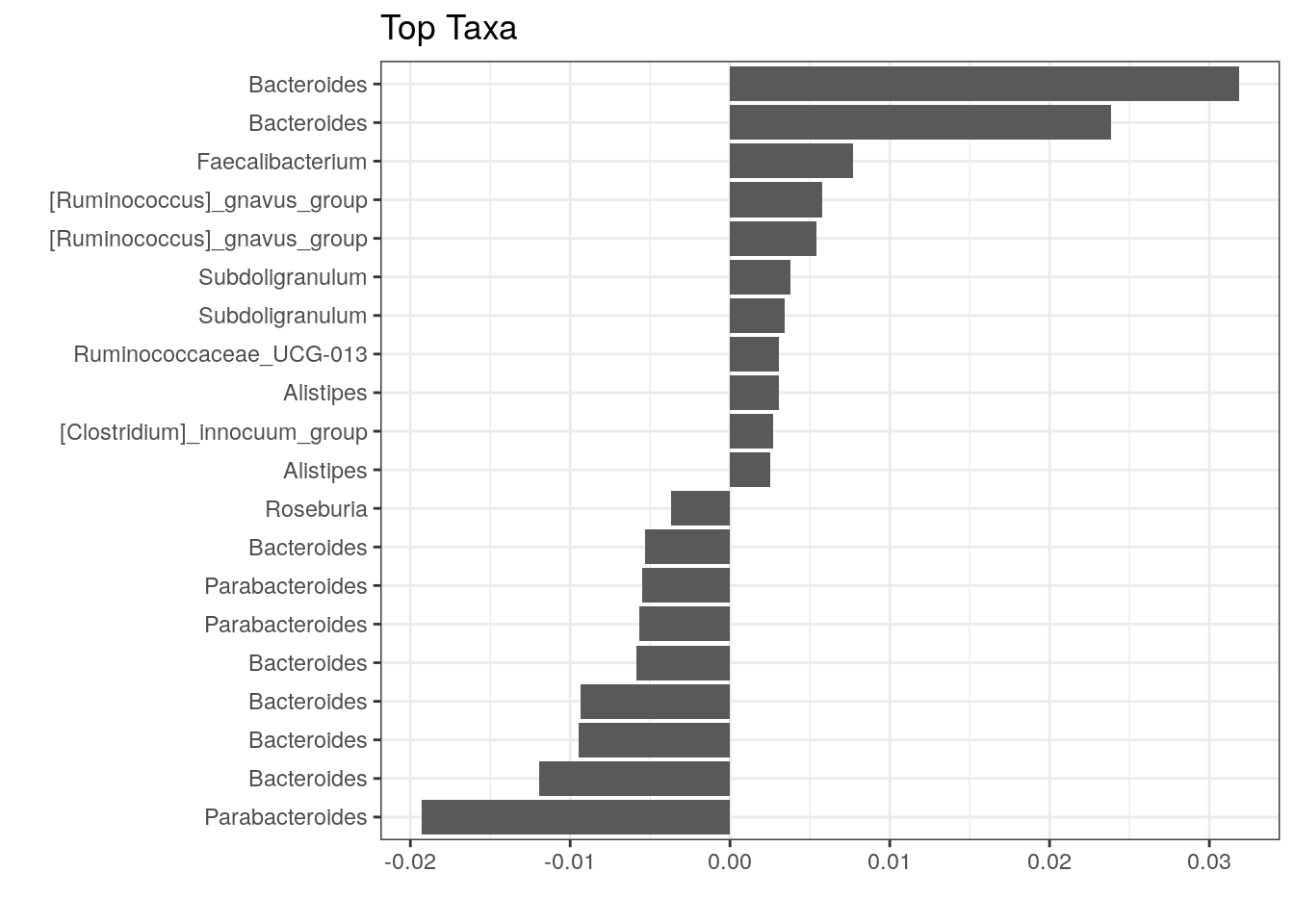
There are many alternative and complementary methods for analysing community composition. For more examples, see a dedicated section on beta diversity in the online book.
8.4 Community typing
A dedicated section presenting examples on community typing is in the online book.
8.5 Exercises
Visualize community variation with different methods (PCA, MDS, t-SNE…) by using the options in the alternative method, plotReducedDim OMA. Compare results obtained with different dissimilarities (Euclidean, Bray-Curtis, Unifrac..) and transformations (CLR, compositional..) of your own choice."
Investigate the influence of the data transformations on statistical analysis: Visualize community variation with PCoA with the following options: 1) Bray-Curtis distances for compositional data; 2) Euclidean distances for CLR-transformed data.
Community-level comparisons: Use PERMANOVA to investigate whether the community composition differs between two groups of individuals (e.g. males and females, or some other grouping of your choice). You can also include covariates such as age and gender, and see how this affects the results.
Perform community typing for the data using the DMM method OMA
Example Solutions
## Loading required package: SummarizedExperiment## Loading required package: MatrixGenerics## Loading required package: matrixStats##
## Attaching package: 'MatrixGenerics'## The following objects are masked from 'package:matrixStats':
##
## colAlls, colAnyNAs, colAnys, colAvgsPerRowSet, colCollapse,
## colCounts, colCummaxs, colCummins, colCumprods, colCumsums,
## colDiffs, colIQRDiffs, colIQRs, colLogSumExps, colMadDiffs,
## colMads, colMaxs, colMeans2, colMedians, colMins, colOrderStats,
## colProds, colQuantiles, colRanges, colRanks, colSdDiffs, colSds,
## colSums2, colTabulates, colVarDiffs, colVars, colWeightedMads,
## colWeightedMeans, colWeightedMedians, colWeightedSds,
## colWeightedVars, rowAlls, rowAnyNAs, rowAnys, rowAvgsPerColSet,
## rowCollapse, rowCounts, rowCummaxs, rowCummins, rowCumprods,
## rowCumsums, rowDiffs, rowIQRDiffs, rowIQRs, rowLogSumExps,
## rowMadDiffs, rowMads, rowMaxs, rowMeans2, rowMedians, rowMins,
## rowOrderStats, rowProds, rowQuantiles, rowRanges, rowRanks,
## rowSdDiffs, rowSds, rowSums2, rowTabulates, rowVarDiffs, rowVars,
## rowWeightedMads, rowWeightedMeans, rowWeightedMedians,
## rowWeightedSds, rowWeightedVars## Loading required package: GenomicRanges## Loading required package: stats4## Loading required package: BiocGenerics##
## Attaching package: 'BiocGenerics'## The following objects are masked from 'package:stats':
##
## IQR, mad, sd, var, xtabs## The following objects are masked from 'package:base':
##
## anyDuplicated, append, as.data.frame, basename, cbind, colnames,
## dirname, do.call, duplicated, eval, evalq, Filter, Find, get, grep,
## grepl, intersect, is.unsorted, lapply, Map, mapply, match, mget,
## order, paste, pmax, pmax.int, pmin, pmin.int, Position, rank,
## rbind, Reduce, rownames, sapply, setdiff, sort, table, tapply,
## union, unique, unsplit, which.max, which.min## Loading required package: S4Vectors##
## Attaching package: 'S4Vectors'## The following objects are masked from 'package:base':
##
## expand.grid, I, unname## Loading required package: IRanges## Loading required package: GenomeInfoDb## Loading required package: Biobase## Welcome to Bioconductor
##
## Vignettes contain introductory material; view with
## 'browseVignettes()'. To cite Bioconductor, see
## 'citation("Biobase")', and for packages 'citation("pkgname")'.##
## Attaching package: 'Biobase'## The following object is masked from 'package:MatrixGenerics':
##
## rowMedians## The following objects are masked from 'package:matrixStats':
##
## anyMissing, rowMedians## Loading required package: SingleCellExperiment## Loading required package: TreeSummarizedExperiment## Loading required package: Biostrings## Loading required package: XVector##
## Attaching package: 'Biostrings'## The following object is masked from 'package:base':
##
## strsplit## Loading required package: ggplot2## Loading required package: ggraph##
## Attaching package: 'dplyr'## The following objects are masked from 'package:Biostrings':
##
## collapse, intersect, setdiff, setequal, union## The following object is masked from 'package:XVector':
##
## slice## The following object is masked from 'package:Biobase':
##
## combine## The following objects are masked from 'package:GenomicRanges':
##
## intersect, setdiff, union## The following object is masked from 'package:GenomeInfoDb':
##
## intersect## The following objects are masked from 'package:IRanges':
##
## collapse, desc, intersect, setdiff, slice, union## The following objects are masked from 'package:S4Vectors':
##
## first, intersect, rename, setdiff, setequal, union## The following objects are masked from 'package:BiocGenerics':
##
## combine, intersect, setdiff, union## The following object is masked from 'package:matrixStats':
##
## count## The following objects are masked from 'package:stats':
##
## filter, lag## The following objects are masked from 'package:base':
##
## intersect, setdiff, setequal, union